EPI-331 for DMD
Duchenne muscular dystrophy (DMD) is a rare and severe X-linked genetic muscle disorder characterized by progressive muscle weakness and degeneration. Symptoms typically begin in early childhood (often between ages 2 and 5 years) and lead to delayed motor milestones, and eventually loss of ambulation by the early teenage years.
It primarily affects boys, with an incidence of ~1 in 3,500 to 5,000 live male births. As the disease progresses, it causes strain in breathing and heart function, making everyday living challenging. Without medical intervention, the average life expectancy is approximately 20–30 years.
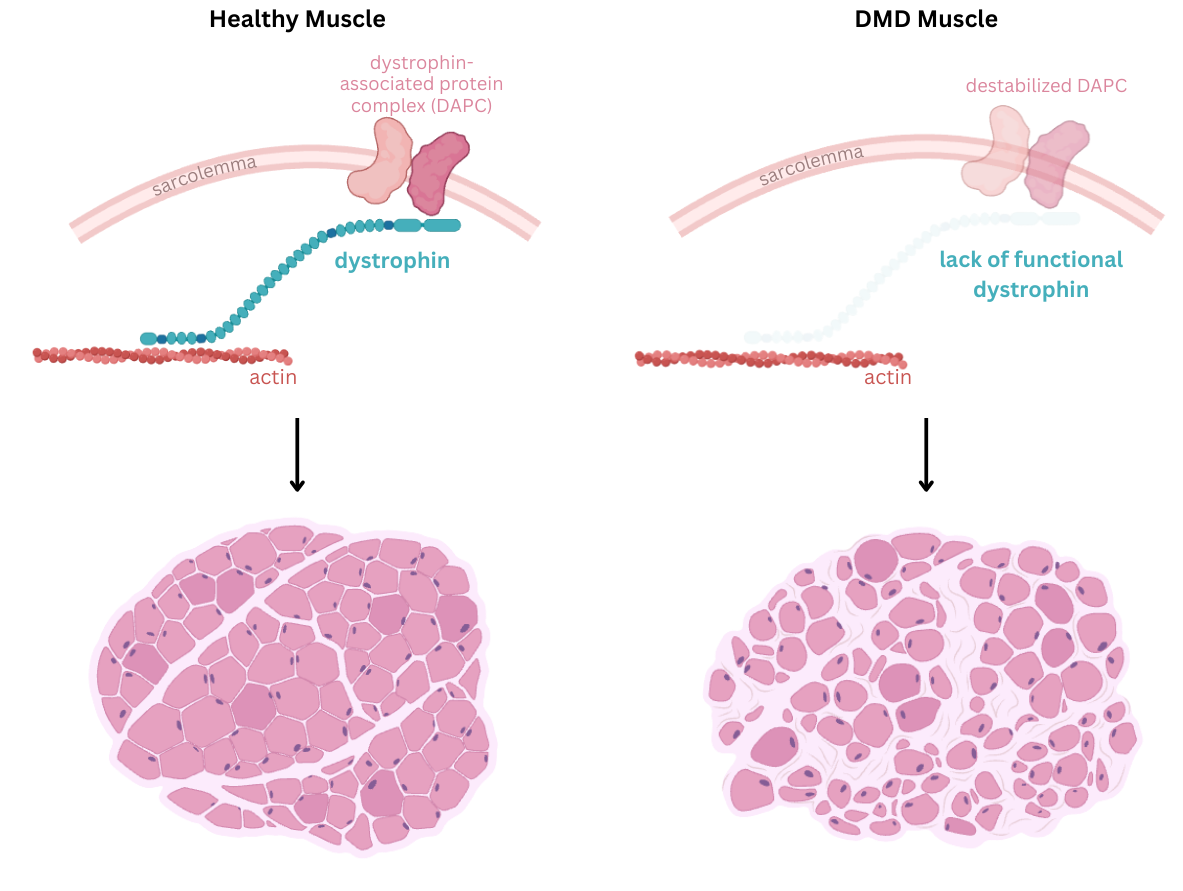
DMD is caused by mutations in the DMD gene located on the X chromosome, which encodes dystrophin: a large, rod-shaped cytoskeletal protein essential for maintaining the structural integrity of muscle cell membrane (sarcolemma) during contraction. A variety of mutations in the dystrophin gene results in loss of dystrophin protein. Without it, the muscle cell membrane becomes fragile and develops repeated tears during normal muscle contractions. Over time, this damage triggers muscle cell death, chronic inflammation, and eventual replacement of muscle tissue with fat and fibrotic tissue. This progressive muscle degeneration severely impairs mobility, breathing, and heart function.
Due to dystrophin’s large size, and the variety of mutations in dystrophin that can cause DMD, existing dystrophin-targeting therapies have faced many limitations. However, Epicrispr’s approach surpasses these limitations by targeting utrophin to treat DMD.
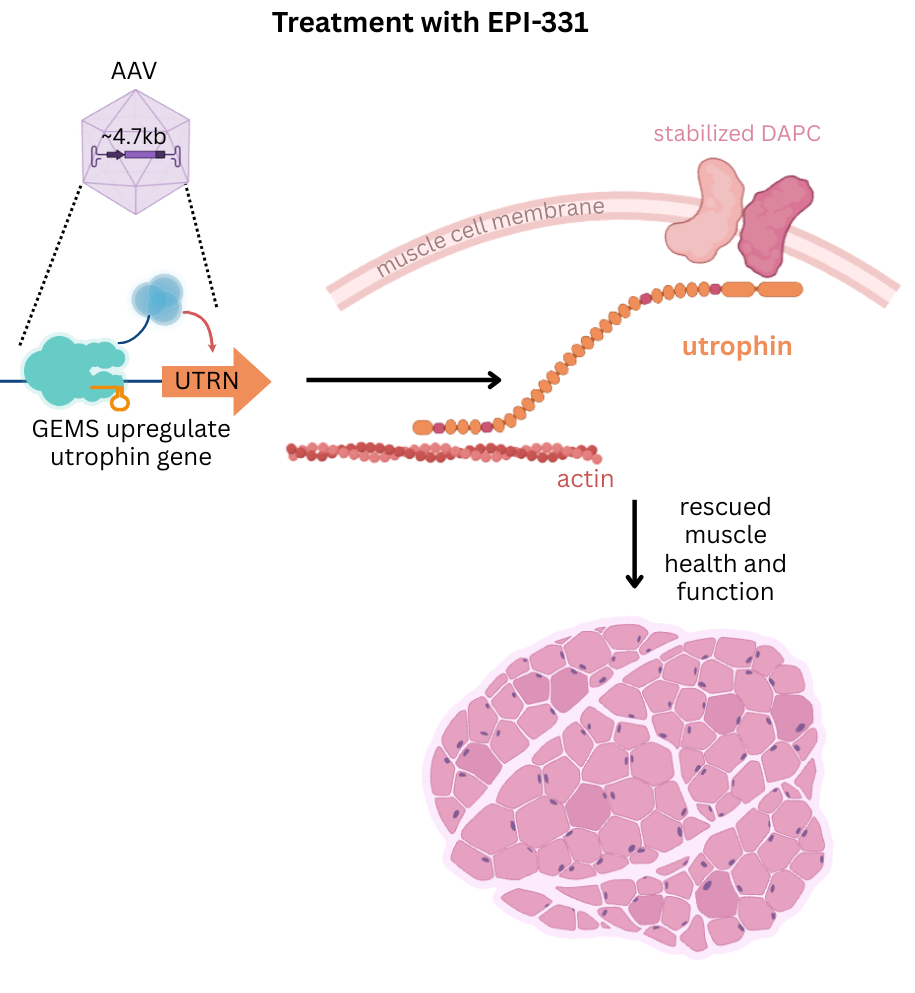
Epicrispr’s proprietary GEMS platform is uniquely suited to induce specific and robust up-regulation of utrophin (UTRN) as a potential therapy to treat DMD. UTRN is a naturally occurring cytoskeletal protein that is structurally and functionally similar to dystrophin. Utrophin is typically expressed during fetal development but after birth, it is downregulated and is functionally replaced by dystrophin. Epicrispr is developing a treatment to selectively activate the UTRN gene, aiming to restore muscle stability and prevent further damage. This UTRN-focused strategy has the potential to benefit a broader population of DMD patients, as it will be an effective treatment in all patients, regardless of their specific Dystrophin mutation. Furthermore, since UTRN is a naturally occurring protein expressed during fetal development, its activation is not expected to trigger an immune response, making it an attractive target for Epicrispr to address the unmet need in DMD patients.
Additional Resources: