EPI-321 for FSHD
Fascioscapulohumeral muscular dystrophy (FSHD) is a genetic muscle disorder that mostly affects the face, scapula, and humerus region. FSHD affects an estimated 870,000 people globally, with no available therapies. Symptoms include chronic pain and loss of movement that usually start before age 20 and may lead to being wheelchair-bound by age 50. FSHD is estimated to be the second most common muscular dystrophy, however, treatment and diagnosis of FSHD is often difficult due to high amounts of variability in disease progression across individuals, and even within different muscles in the same patient. Thus, FSHD patients have a large unmet need for curative treatments and deeper understanding of the disease as a whole.
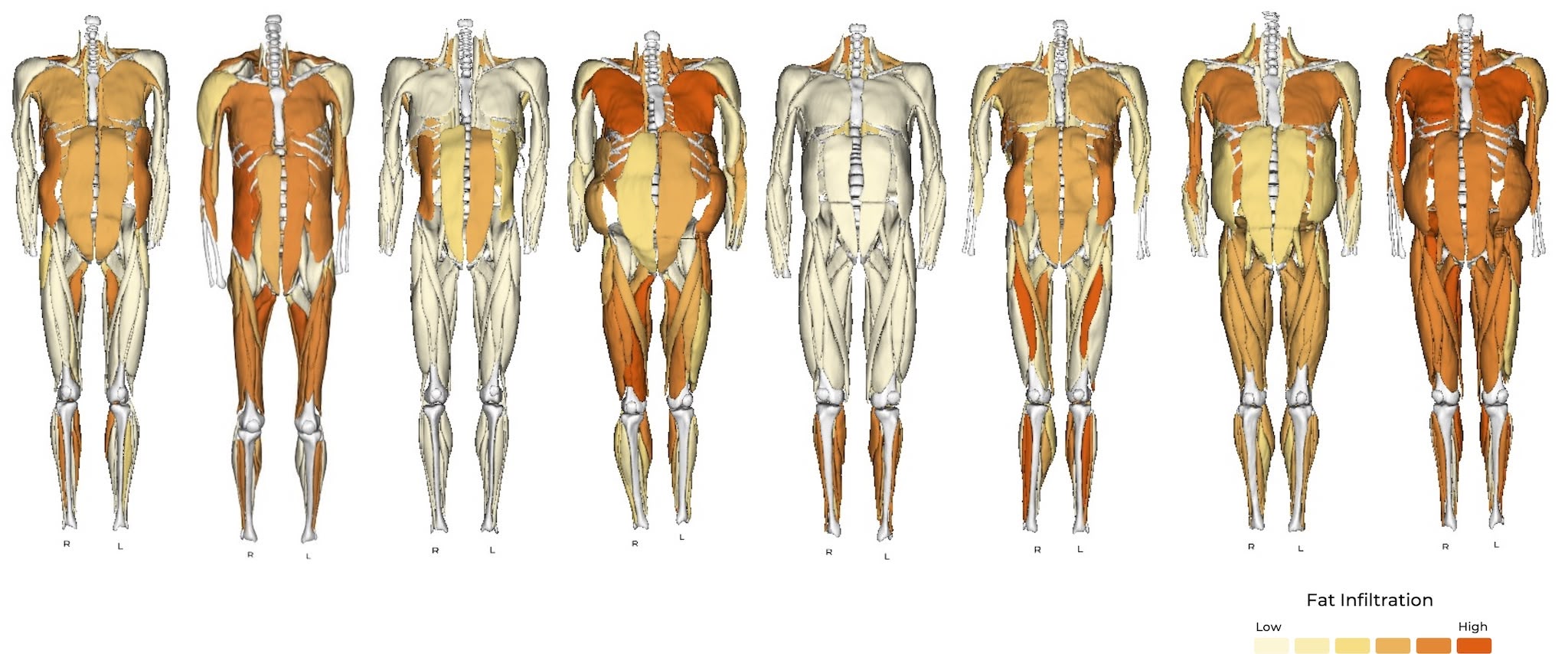
Variety of muscle phenotypes displayed by FSHD patients – Image from Springbok Analytics
FSHD is caused by hypomethylation of the D4Z4 array, often driven by array contraction or by genetic deletions in the region. This hypomethylation leads to sporadic expression of DUX4, a gene that is typically only expressed during embryogenesis.
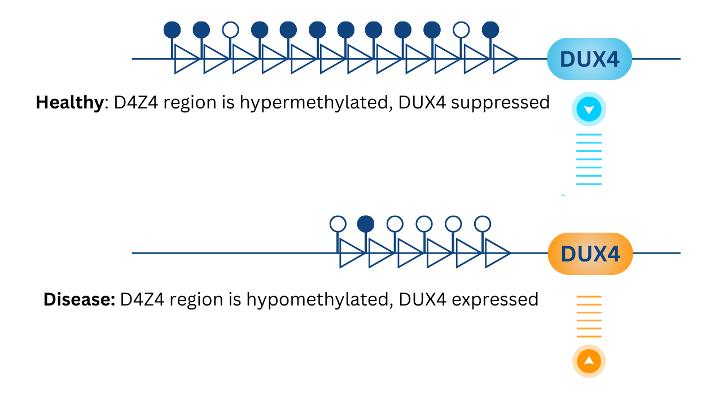
Aberrant DUX4 expression induces cell death and tissue degeneration in the muscle, leading to the progressive weakening of skeletal muscle tissues seen in FSHD patients. This weakening can lead to muscle asymmetry and fat infiltration, as seen in a full body MRI scan of an FSHD patient performed by Springbok Analytics.
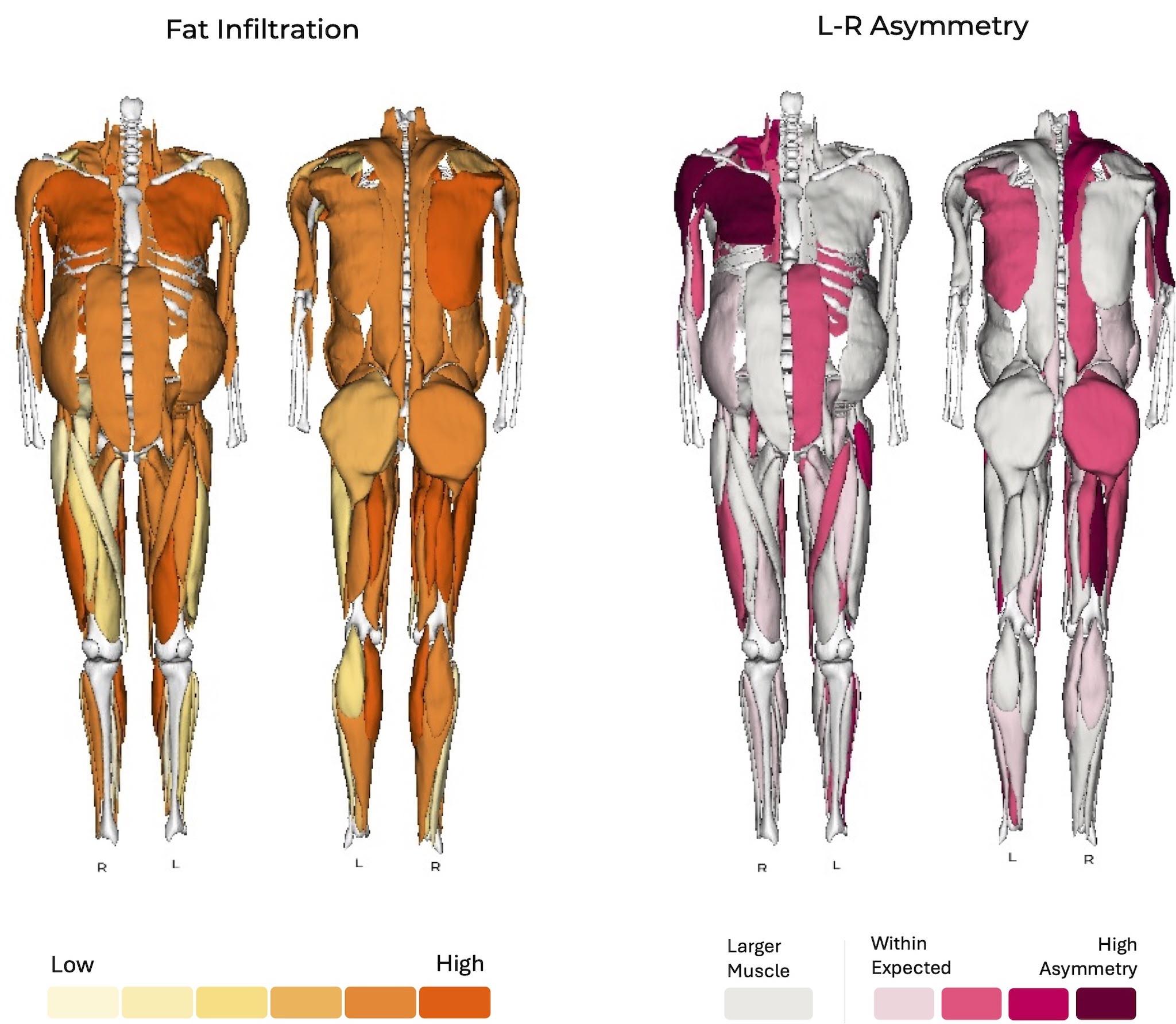
Fat Infiltration and Left-Right Asymmetry – Image from Springbok Analytics
To address the unmet need in FSHD patients, Epicrispr has developed EPI-321, an adeno-associated virus (AAV) delivered epigenetic gene therapy that re-methylates the D4Z4 region in skeletal muscle.
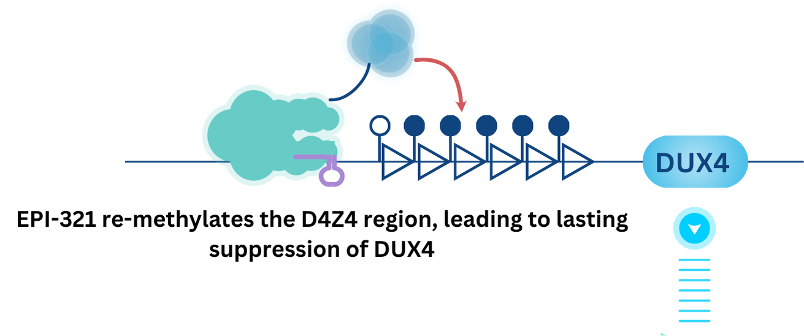
This one-time dose of EPI-321 is directed to muscle tissue within a single AAV vector, which has been clinically validated for muscle delivery. Preclinical data collected from in vitro culture systems and in vivo animal models demonstrated strong efficacy: including robust suppression of the DUX4 transcript and re-methylation of the D4Z4 array. In addition, treatment with EPI-321 resulted in improved muscle contractility and strong reduction in muscle cell death.
Epicrispr has initiated a clinical trial in 2025, for more information, please visit https://www.clinicaltrials.gov/study/NCT06907875. For patients interested in the study, please see here for more information and contact the trial sites as they are listed on the clinicaltrials.gov website.
Additional Resources: